For many Canadians with neuromuscular disorders, the journey to diagnosis is long and frustrating, marked by uncertainty, misdiagnoses, and delays. Myotonic Dystrophy Type 1 affects not only mobility but also the heart, cognition, and the gastrointestinal system, yet many individuals spend years searching for answers before finally accessing genetic testing. These delays result in missed opportunities for early intervention, symptom management, family planning, and access to clinical trials and emerging treatments.
Recognizing the need for earlier, more equitable access, Muscular Dystrophy Canada has launched a nationwide initiative to improve genetic testing for Myotonic Dystrophy.
A timely and accurate diagnosis is more than just an answer – it unlocks specialized care, clinical trials, and innovative treatments that could slow disease progression and improve quality of life. Through a landscape analysis, Muscular Dystrophy Canada is identifying testing barriers, reimbursement challenges, and long wait times while at the same time offering no-cost genetic testing and counselling for Canadians with a suspected diagnosis or family history of Myotonic dystrophy.
Addressing Delays in Treatment Access
Canadians face delays due to regulatory approvals and reimbursement barriers. Even after Health Canada approves a therapy, provincial funding decisions can take months or even years, leaving patients waiting for treatments that could preserve mobility, independence, and quality of life.
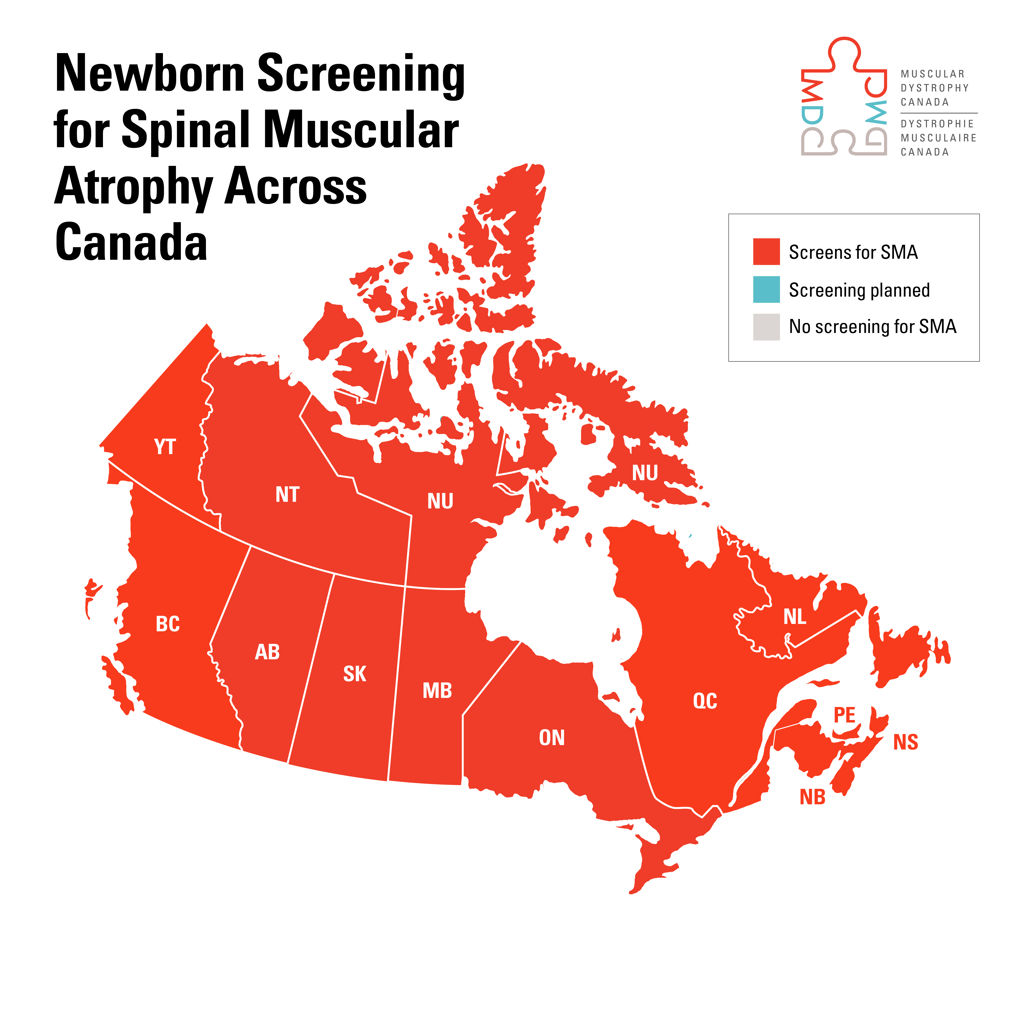
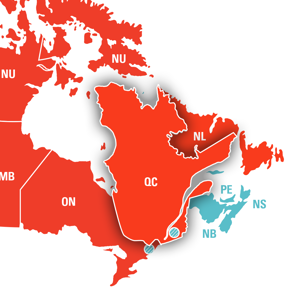
Duchenne muscular dystrophy, for example – approved treatments exist in other countries, yet none are available in Canada. Individuals with Myasthenia Gravis face a different challenge – drugs approved by Health Canada remain inaccessible because they are not yet covered by provincial drug plans. Meanwhile, access to spinal muscular atrophy treatments is inconsistent across Canada, with adults in Quebec eligible for therapy while those in other provinces are denied.
Muscular Dystrophy Canada continues to advocate for faster access to diagnosis, treatment approvals, and reimbursement policies that put persons impacted first.
Breaking Down Financial Barriers: Ensuring Access to Essential Equipment
For individuals with neuromuscular disorders, mobility aids, assistive devices and medical equipment are essential to safety, independence, and quality of life. Yet, Hoyer lifts, wheelchairs, hospital beds, and pressure-relieving mattresses – critical for mobility, caregiver safety and daily living – are not consistently covered by provincial health programs and or private insurance.
Without adequate funding, many individuals are left paying out of pocket, waiting years for partial assistance, or simply going without. Thanks to generous donors, Muscular Dystrophy Canada’s Equipment Program helps bridge some gaps, but demand far exceeds available resources. Through #YourDevicesYourRights, Muscular Dystrophy Canada is working with like-minded community partners advocating for improvements in funding with provincial programs.
While governments cannot cover everything, some equipment – like Hoyer lifts or wheelchair adaptations – is essential. No one should be denied access because of where they live or their financial situation. Ensuring consistent, needs-based funding across Canada is a matter of dignity, safety, and human rights.
Through your support of Muscular Dystrophy Canada, you are making an impact. Join us in breaking down barriers to ensure that all Canadians with neuromuscular disorders receive the diagnosis, treatment, and support they need – when they need it.