FOR IMMEDIATE RELEASE March 22, 2023
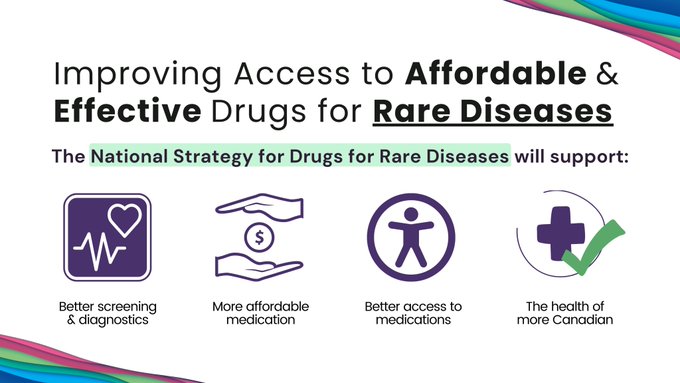
Toronto, Ontario – Muscular Dystrophy Canada applauds the Government of Canada for committing $1.5 billion over three years in support of a National Strategy for Drugs for Rare Diseases to help increase access to, and affordability of, promising and effective drugs for rare diseases to improve the health of patients across Canada.
“MDC works with Canadian Fire Fighters, donors, volunteers and other like-minded organizations to break down barriers for the neuromuscular community. The major barriers that exist for our diverse community, which consists of individuals with rare and ‘ultra-rare’ neuromuscular disorders, are lack of cures and delayed – out of reach treatments. So this announcement is great news for our community,” said Stacey Lintern, CEO, Muscular Dystrophy Canada. She added, “we are excited that this announcement has been made but time is of the essence for many rare diseases and the sooner we can move this from strategy to action, the better it is for our community.”
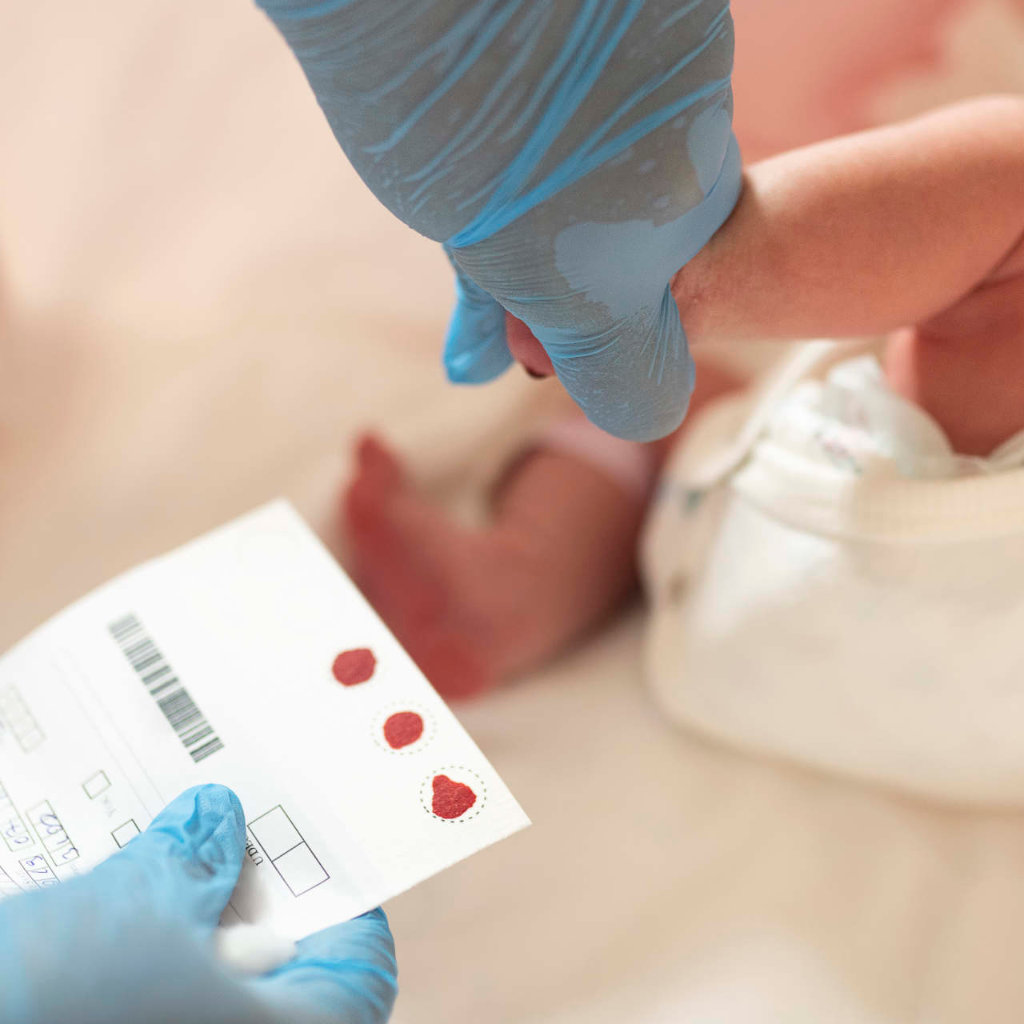
This long-awaited announcement identified four areas of focus for the national strategy and funding support for provinces and territories to improve access to new and emerging drugs, enhance access to existing drugs, early diagnosis, and screening for rare diseases.
“The areas of focus align perfectly with the work that MDC is already doing such as ensuring early diagnosis and clinical genetic testing. We know access to an accurate diagnosis, coupled with early access to treatments, are important for optimal outcomes and better quality of life,” said Lintern.
“MDC welcomes a strategy where the patient and family are at the forefront and that supports an evidence-based care journey. We are committed to working with our partners like the Neuromuscular Disease Network for Canada (NMD4C) to help make that happen, and bring about better diagnostic tools, support for clinical trials and evidence that supports decision-making. We look forward to a time when the neuromuscular community has access to accurate and early diagnoses, life-changing treatments, and supports regardless of where they live, their age or the rarity of the condition.”
The full Government of Canada announcement can be read here
-30-
ABOUT MUSCULAR DYSTROPHY CANADA
Muscular Dystrophy Canada’s mission is to enhance the lives of persons affected by neuromuscular disorders by continually working to provide ongoing support and resources while relentlessly searching for cures through well-funded research. To learn more about MDC, please visit muscle.ca or call our toll-free number at 1-800-567-2873.
FOR MORE INFORMATION CONTACT:
Heather Rice
Muscular Dystrophy Canada
Heather.Rice@muscle.ca
902-440-3714