FOR IMMEDIATE RELEASE – Muscular Dystrophy Canada (MDC) is thrilled to announce that spinal muscular atrophy (SMA) has been added to the newborn screening panel in Quebec. A significant milestone for the province and MDC.
“Congratulations to the Government of Quebec on this step, which will lead to early diagnosis and treatments that will have life-changing results for individuals and families,” said Stacey Lintern, CEO, Muscular Dystrophy Canada.
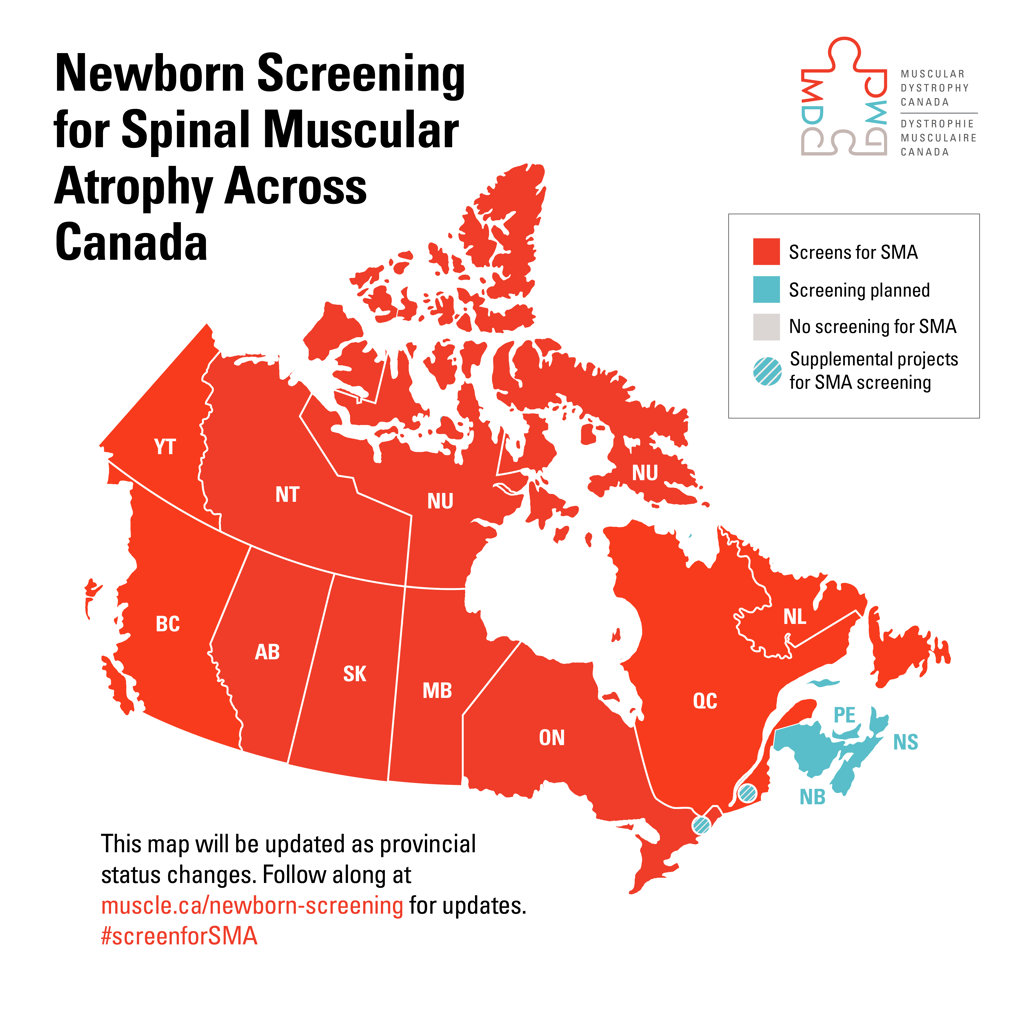
Screening is being integrated now, with full implementation to take place by the end of 2023.
Over the past two years, MDC collaborated with Novartis Pharmaceuticals Canada Inc. as well as clinicians, researchers, people affected by SMA and families, Fire Fighters and donors to advocate for the inclusion of SMA on the newborn screening panel.
Extensive conversations, collaboration and financial contributions ($583,778) towards the lab program at CHU de Québec-Université Laval, equipment, and building an evidence-based pathway for the rapid early initiation of disease-modifying therapy for individuals with SMA across Quebec, led to this exciting change in policy.
“It was important for MDC to go beyond supporting the necessary infrastructure for SMA newborn screening, and to also ensure families receive the best quality care in the event of a positive screening result,” added Lintern. “We couldn’t be happier with the recent news. The addition of SMA to the panel means that infants and families in Quebec will now equitably benefit from the same opportunities as the majority of other Canadians, gaining access to early detection possibly before the onset of symptoms.”
MDC is currently working with Maritime provinces to begin the implementation of newborn screening for SMA. For more information on MDC’s efforts in ensuring all Canadian babies are screened for SMA, visit muscle.ca/services-support/newborn-screening/.
– 30 –
For more information, contact:
Sylvie St-AmandTranslation and Communication Specialist
Muscular Dystrophy Canada
Phone: 514-244-0381
Email: sylvie.st-amand@muscle.ca








 Stacey Lintern
Stacey Lintern Perry Esler
Perry Esler