FOR IMMEDIATE RELEASE
Toronto, Ontario – Spirits are high as Canadians from coast to coast to coast get ready to walk and roll starting May 11 to raise funds and break down barriers in support of their friends, families and neighbours affected by neuromuscular disorders (NMDs).
The Walk and Roll for Muscular Dystrophy Canada (MDC) takes place in more than 30 communities across the country from May to October to raise critical funds to break down barriers for the neuromuscular disorder community, and ultimately find cures. Due to interest, events in Burnaby and Surrey, BC have been combined into one bigger celebration and new events have been added in Grande Prairie, AB and Sherbrooke, QC.
The goal is to raise $1.3 million.
“The Walk and Roll for MDC is a critical fundraising event. The funds raised by participants are invested in ground-breaking research, ensuring Canada is prepared to provide access to new life-changing treatments, while also filling the immediate need for treatment and care options,” said Stacey Lintern, CEO, Muscular Dystrophy Canada.
Dr Jodi Warman Chardon, Clinical Scientist in the Department of Medicine at the Ottawa Hospital added, “For many disorders, there isn’t a treatment yet, but MDC-funded research gives people true hope. We’ve made incredible discoveries in the last few years, and they’re just going to accelerate.”
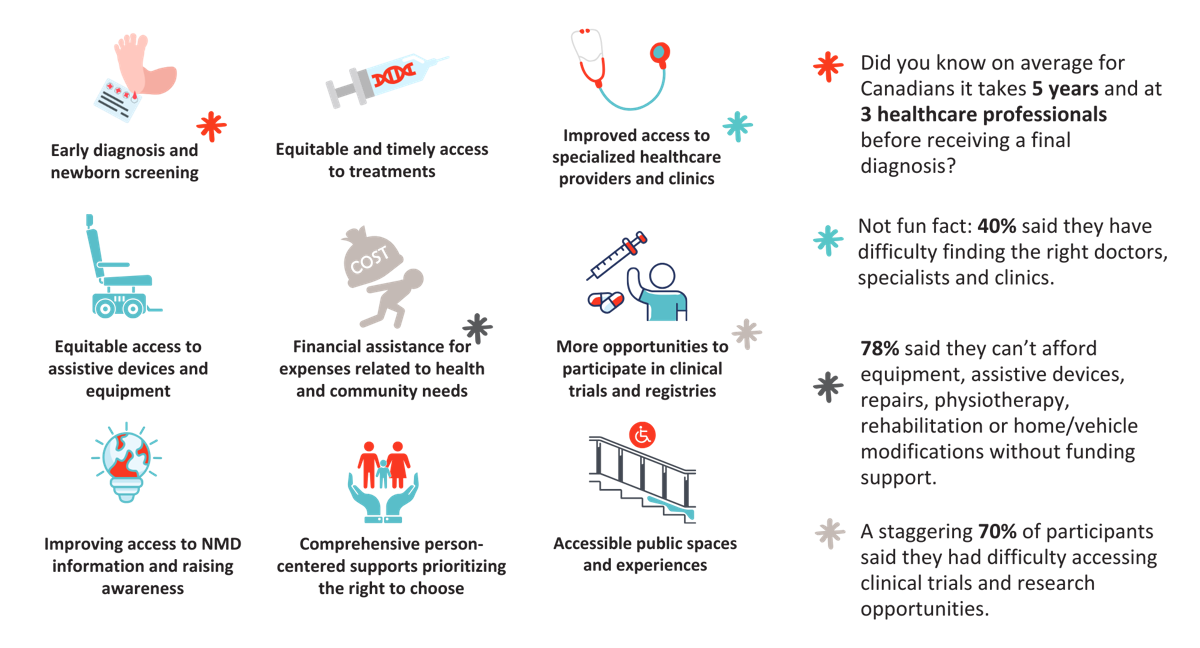
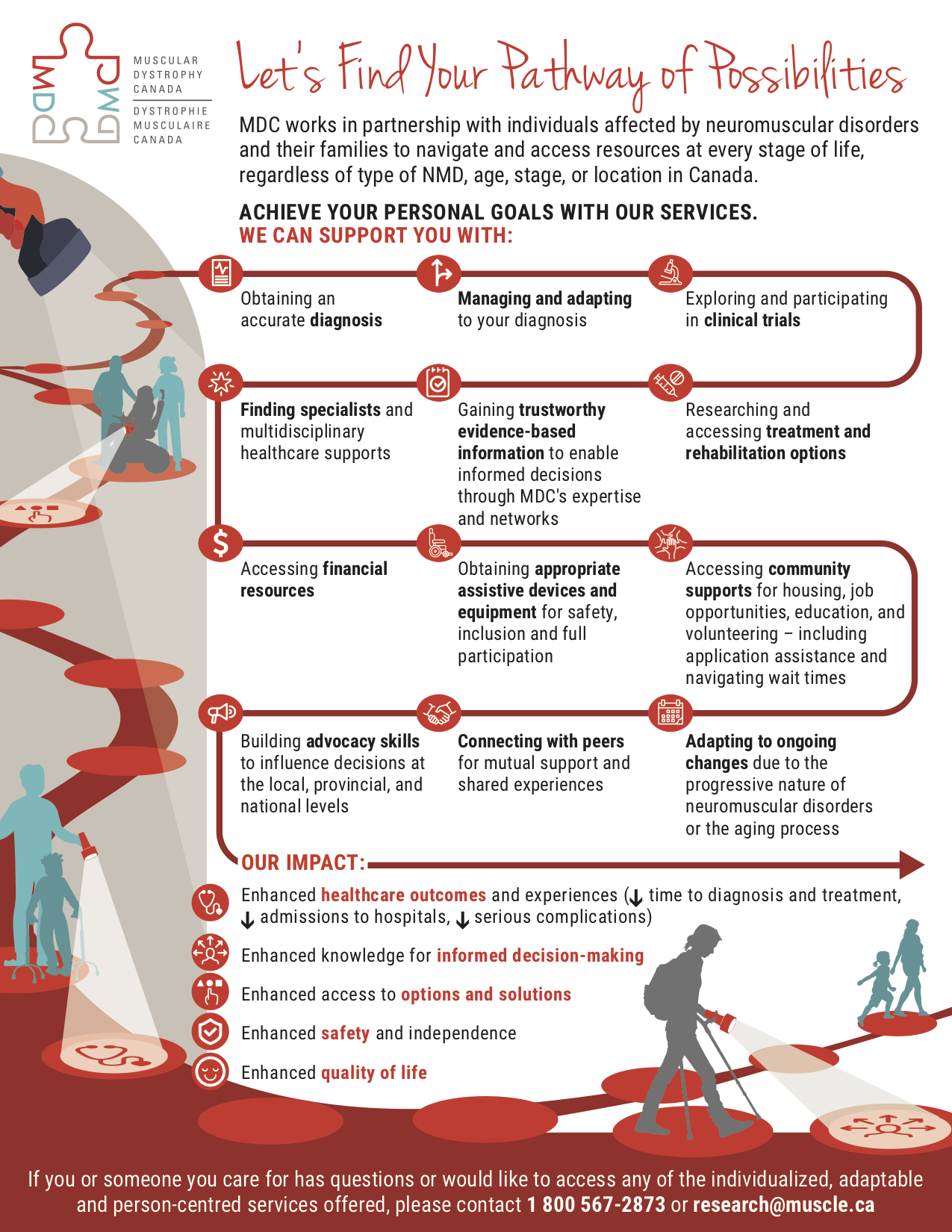
Lintern continued, “Walk and Roll for MDC fundraising ensures that when someone needs vital equipment to help live their best life – or even save their life – they won’t have to struggle. Every dollar raised helps MDC deliver a variety of services for all Canadians while also raising the neuromuscular community’s voice and improving access to key decision-makers who can address gaps in the healthcare system.”
But Walk & Roll for MDC is more than a fundraiser. It is a fully accessible community event that is fun for families, friends, businesses and colleagues. It’s a chance for individuals and families to break down barriers together, connect with others who have similar experiences and share their learnings and words of support. It’s a chance for Fire Fighters to connect with the families they help all year long and for members of the research community to hear about individuals who have accessed treatment or therapies.
When you register or donate to the Walk and Roll for MDC, you are breaking down barriers for thousands of Canadians right now – while also giving hope to future generations. For more information on locations, to donate or register visit, WalkRollMDC.ca.
– 30 –
ABOUT MUSCULAR DYSTROPHY CANADA
Muscular Dystrophy Canada’s mission is to enhance the lives of those affected by neuromuscular disorders by continually working to provide ongoing support and resources while relentlessly searching for cures through well-funded research. To learn more about Muscular Dystrophy Canada, visit muscle.ca or call 1-800-567-2873.
FOR MORE INFORMATION CONTACT:
Heather RiceDirector, Marketing & Communications
Heather.Rice@muscle.ca
902-440-3714