Toronto, Ontario, Canada, January 27, 2025 – Muscular Dystrophy Canada is thrilled to launch an innovative initiative designed to tackle the diagnostic hurdles experienced by individuals with myotonic dystrophy. This transformative, year-long project will offer no-cost genetic testing to those with suspected myotonic dystrophy, providing a critical pathway to earlier and more accurate diagnoses. Alongside genetic testing, Muscular Dystrophy Canada will also provide access to post-test genetic counseling, empowering individuals to better understand their results and make informed decisions about their health and future clinical care.
“The journey to a diagnosis is often long and filled with barriers for individuals living with neuromuscular disorders, and this is especially true for those with myotonic dystrophy,” says Dr Homira Osman, Vice-President of Research and Public Policy at Muscular Dystrophy Canada. “Without a confirmed diagnosis, patients face significant uncertainty—not only about what is happening to them but also about how their condition might be treated. Access to timely and accurate genetic testing changes that. It empowers individuals with a clear understanding of their condition, enabling them to make informed choices about their care, explore opportunities for emerging treatments and clinical trials, and manage care. A confirmed diagnosis is the cornerstone of better health outcomes and improved quality of life.”
Myotonic dystrophy is a genetic disorder that affects muscles and other body systems, causing symptoms such as muscle weakness, difficulty relaxing muscles (myotonia), and, in some cases, problems with the heart, eyes, and cognitive function. There are two main types of myotonic dystrophy: Type 1 (DM1) and Type 2 (DM2), both caused by mutations in specific genes. With over 1,200 individuals affected by DM registered with Muscular Dystrophy Canada, it is the most prevalent neuromuscular disorder in Muscular Dystrophy Canada’s database.
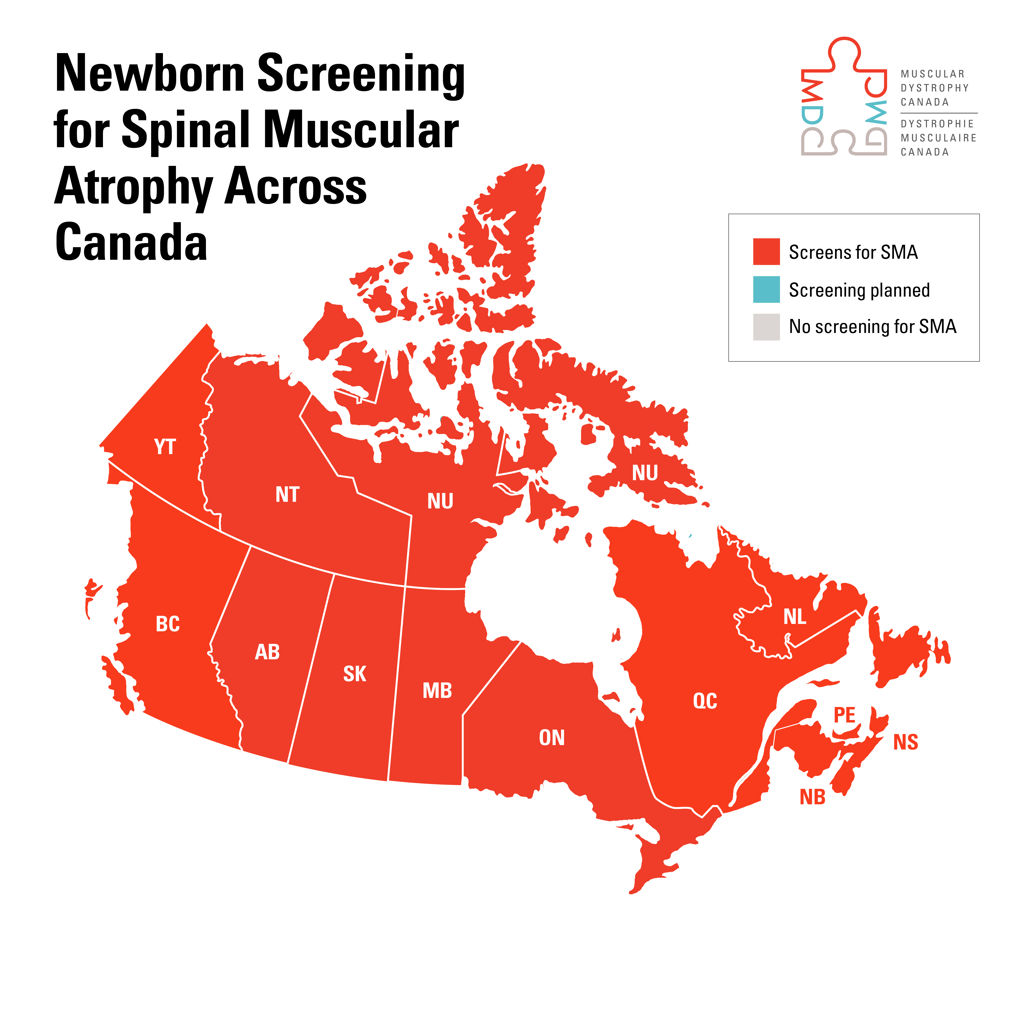
Stacey Lintern, CEO of Muscular Dystrophy Canada says, “Muscular Dystrophy Canada is uniquely positioned to lead this initiative, leveraging its proven track record in expanding newborn screening for spinal muscular atrophy nationwide. With established partnerships across healthcare, research, and public policy, Muscular Dystrophy Canada has the expertise to drive meaningful impact and support early and accurate diagnoses for Canadians affected by myotonic dystrophy.”
“My family’s journey with myotonic dystrophy has taught me that knowledge is power,” says Teresa Buffone of Ontario, whose son is affected by myotonic dystrophy. “When my son needed surgery, a confirmed diagnosis from genetic testing made a world of difference to the care he received. Patients with myotonic dystrophy can react badly to general anesthesia, leading to complications that can be deadly. Having a confirmed diagnosis empowered me to speak with the surgeon and ensure that he would receive the care that was right for him – a confidence that could very well have saved his life. Genetic testing gave us the answers we needed and has enabled us to face the challenges ahead with courage.”
“As a family affected by myotonic dystrophy, we’re filled with hope knowing that treatments are finally becoming a reality,” says the Leboeuf family, of Ontario. “Clinical trials and innovative medicines hold so much promise, but they require a confirmed diagnosis to participate. While this is an exciting time, we also recognize how devastating it can be for those who remain undiagnosed, as it creates a significant barrier to accessing these life-changing advancements sooner. Ensuring access to timely and accurate diagnoses is essential for unlocking opportunities and giving everyone a chance at better outcomes.”
Stacey continues, “At Muscular Dystrophy Canada, we believe that access to genetic testing is a fundamental right of Canadians affected by a neuromuscular disorder and we are grateful for the partnership of the neuromuscular community for helping to make this life-changing project a reality. With support from clinicians, researchers, healthcare practitioners, volunteers, donors, Canadian Fire Fighters, like-minded organizations, and sponsors, this project is a significant step forward into breaking down the barriers that keep Canadians affected by myotonic dystrophy from living their best lives.”
If you or someone you know is interested in receiving genetic testing for myotonic dystrophy, please reach out to Muscular Dystrophy Canada at research@muscle.ca or 1-800-567-2873, Ext. 5401.
– 30 –
ABOUT MUSCULAR DYSTROPHY CANADA
Muscular Dystrophy Canada’s mission is to enhance the lives of those affected with neuromuscular disorders by continually working to provide ongoing support and resources while relentlessly searching for a cure through well-funded research. To learn more about Muscular Dystrophy Canada, please visit muscle.ca or call our toll-free number at 1-800-567-2873.
FOR MORE INFORMATION CONTACT:
Homira OsmanVice-President, Muscular Dystrophy Canada
Homira.Osman@muscle.ca
1-800-567-2873 ext. 9037





 Dr Haim Abenhaim
Dr Haim Abenhaim Dr Krista Best
Dr Krista Best Dr Nathalie Bier
Dr Nathalie Bier Dr Rageen Rajendram
Dr Rageen Rajendram Dr Karine Choquet
Dr Karine Choquet Dr Lisa Hoffman
Dr Lisa Hoffman Dr Rashmi Kothary
Dr Rashmi Kothary Dr Keir Menzies
Dr Keir Menzies Dr Gerald Pfeffer
Dr Gerald Pfeffer