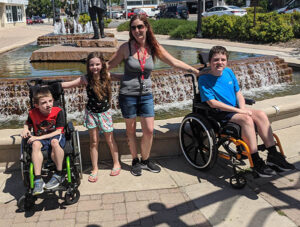New partnership offers access to shared decision making support for paediatric clients and their families in Ontario
For Immediate Release – June 23, 2020
Ontario, Canada – Muscular Dystrophy Canada (MDC), Children’s Hospital at London Health Sciences Centre (LHSC), and Children’s Health Foundation have entered into a partnership to offer a pilot project providing access to an innovative service that helps paediatric patients and their families make informed decisions throughout their care journey. Through this partnership, Ontario-based MDC clients up to age 18 and their parents and caregivers will have access to virtual Shared Decision Making coaching from Children’s Hospital via Ontario Telemedicine Network (OTN) Technology.
Shared Decision Making is the process where health professionals and patients collaborate on medical decision-making with a more structured method that takes into account both the best evidence and patient values. Health-related decisions can affect the overall well-being and quality of life of both the patient and their family members, and Shared Decision Making has been shown to reduce these psychosocial stressors. The decision coaching provided by Children’s Hospital at LHSC is particularly innovative because it involves unbiased decision support from outside the patient’s circle of care, filling a unique gap that is present in models where only practitioners are directly involved in the decision coaching. For children and youth with neuromuscular disorders and their families, this innovative model becomes all the more valuable as there are many challenging medical and non-medical decisions that must be made over a patient’s health-care journey.
“We are so excited about this collaborative partnership and the benefits our Ontario clients will gain from the Shared Decision Making program. Having support from a Decision Coach will help alleviate some of the fear, stress and pressure parents of children with neuromuscular disorders often experience,” says Stacey Lintern, Interim CEO, Muscular Dystrophy Canada. “This program will also support clients and families in gaining knowledge, evaluating benefits and drawbacks and clarifying priorities which are all integral to a comprehensive approach to making challenging medical decisions. MDC Service Specialists are happy to be able to offer this program as an additional resource to clients as they work with them to navigate and access critical supports.”
The Shared Decision Making partnership is in line with MDC’s commitment to client and family-centred care. MDC currently takes a client and family-centred approach to delivering its mission across Canada by offering a Systems Navigation program, which provides critical support not only in ensuring clients have access to the right resources but also by playing a key role in providing education, developing networks and connections, working in partnership to address barriers and share resources, enhancing life skills and self-coping strategies and embracing inclusion. The Shared Decision Making program includes education, tools, strategies and information. The partnership will provide MDC clients with a seamless process, allowing them to access the right resources at the right time.
“We are incredibly pleased to be able to partner with Muscular Dystrophy Canada to extend our decision coaching service to other patients and families across Ontario,” says Dr. Craig Campbell, Interim Chair of Paediatrics at Children’s Hospital at LHSC. “The Children’s Hospital team, with the support of our Children’s Health Foundation, has been able to develop a unique expertise in this area over the last two-years, and engaging in this new partnership is a rewarding recognition of that. We look forward to furthering our support of Ontario patients and families as they make important care decisions about their child’s neuromuscular disorder.”
The province-wide pilot project aims to collect data that will demonstrate the need for broader investment into a systemic-approach to the availability of decision coaching for all patients and families impacted by neuromuscular disorders across Canada.
Access support now.
– 30 –
ABOUT MUSCULAR DYSTROPHY CANADA
Muscular Dystrophy Canada’s mission is to enhance the lives of those impacted with neuromuscular disorders by continually working to provide ongoing support and resources while relentlessly searching for a cure through well-funded research. To learn more about Muscular Dystrophy Canada, please visit www.muscle.ca or call our toll-free number at 1-800-567-2873.
ABOUT LONDON HEALTH SCIENCES CENTRE
London Health Sciences Centre has been at the forefront of medicine in Canada for 145 years and offers the broadest range of specialized clinical services in Ontario. Building on the traditions of its founding hospitals to provide compassionate care in an academic teaching setting, London Health Sciences Centre is home to Children’s Hospital, University Hospital, Victoria Hospital, the Kidney Care Centre, two family medical centres, and two research institutes – Children’s Health Research Institute and Lawson Health Research Institute. As a leader in medical discovery and health research, London Health Sciences Centre has a history of over 70 international and national firsts and attracts top clinicians and researchers from around the world. As a regional referral centre, London Health Sciences Centre cares for the most medically complex patients including critically injured adults and children in southwestern Ontario and beyond. The hospital’s nearly 15,000 staff, physicians, students and volunteers provide care for more than one million patient visits a year. For more information visit www.lhsc.on.ca
ABOUT CHILDREN’S HEALTH FOUNDATION
Children’s Health Foundation, founded in 1922, raises funds to ensure that children and their families across Western Ontario receive the best possible care and the most possible hope when faced with a life-threatening or life-limiting diagnosis. By funding equipment, programs and research at Children’s Hospital at London Health Sciences Centre, Thames Valley Children’s Centre and Children’s Health Research Institute, Children’s Health Foundation ensures better childhoods for kids facing serious health issues, and hope, relief and support for those who love them. To learn more, visit childhealth.ca.
Media Contact
Heather Rice
Muscular Dystrophy Canada
Heather.Rice@muscle.ca
902-440-3714