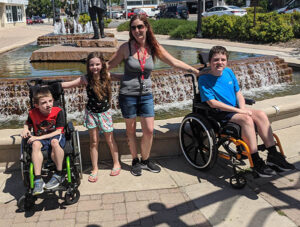SMA & Related Disorders | “Mighty Max” Sych
“Max was diagnosed with SMA Type 2 during the pandemic.  We have had to learn to be patient with each other when navigating this new way of life. We have spent hours on Zoom calls with physiotherapists, occupational therapists, and kinesiologists in order to get Max moving. There were many times, we wished we could just be there in person. It has been hard but extremely rewarding when the person on the other side of the screen tells you how well Max is doing!
We have had to learn to be patient with each other when navigating this new way of life. We have spent hours on Zoom calls with physiotherapists, occupational therapists, and kinesiologists in order to get Max moving. There were many times, we wished we could just be there in person. It has been hard but extremely rewarding when the person on the other side of the screen tells you how well Max is doing!
Throughout the pandemic we have also been advocating for access to life-changing treatment for Max. As a parent you are your child’s number one advocate and although the pandemic made it that much more difficult, it has been so important for us to never give up the fight.”
- Bryarly Parker and Bowden Sych, parents of Max.
Congenital Myopathies | Susan Jahnke
“I have a rare neuromuscular disorder that most people understandably haven't heard of,  congenital fibre type disproportion. I have good days where I don't feel too bad, and don't think about my disability much - but I haven't had days like that during the pandemic. I'm confronted by being 'vulnerable' every time I read the news or try to plan even a very limited outing. It's inescapable, this reminder of how susceptible I am to complications of COVID-19, and it's felt very scary and isolating to have to be so very careful.
congenital fibre type disproportion. I have good days where I don't feel too bad, and don't think about my disability much - but I haven't had days like that during the pandemic. I'm confronted by being 'vulnerable' every time I read the news or try to plan even a very limited outing. It's inescapable, this reminder of how susceptible I am to complications of COVID-19, and it's felt very scary and isolating to have to be so very careful.
I miss seeing my friends so much, I miss seeing my mum and my brother... but the hardest thing about life during the pandemic is some of the tough choices my family has had to make. My stepdaughter is in high school, and trying to balance keeping me safe and keeping her life as normal as possible with her education and her work, has meant that she's spending most of her time with her other parent. This has been such a painful and ongoing balancing act.
I also have to choose between getting the medical and health care I need, and risking a COVID-19 exposure. Which is more important? It's such a hard thing to figure out and my general health has really been suffering. I'm glad I'm able to stay in contact with my GP over the telephone, but I'm missing out on critical care like physiotherapy and specialists.
One silver lining of the pandemic is how much it's highlighted the need for connection and I try to find new ways of reaching out, even on the hard days.”
- Susan Jahnke
Genetic and Immune-Mediated Neuromuscular Junction Disorders | Clinton Peres
“I lost both my parents and my aunt to COVID-19. What’s been even more heartbreaking is the  fact that I was not able to see them when they were sick. This was not only because of the restrictions in my province, but because I have a rare condition called Myasthenia Gravis, which puts me at risk for possible severe COVID-19 complications.
fact that I was not able to see them when they were sick. This was not only because of the restrictions in my province, but because I have a rare condition called Myasthenia Gravis, which puts me at risk for possible severe COVID-19 complications.
The pandemic has made life living with Myasthenia Gravis very challenging. I have always been very close to my family and they have been there for me on my hard days. I still remember when my mom held my hand when I received my diagnosis. It is heartbreaking that I was not there in her final days to support her.
COVID-19 and has taught me the role of family when living with a rare neuromuscular disorder. This pandemic has been hard for all of us – we’ve been forced to stay at home, isolate and wear masks– but keep in mind these precautions are way better than being alone without loved ones in the final moments of your life. Let’s all continue to stay safe not only for ourselves, family members but also for the neuromuscular disease community.”
- Clinton Peres
Metabolic Myopathies | Brad Crittenden
“Living with a neuromuscular disorder, in my case Pompe Disease, in the midst of the COVID-19 pandemic has made healthcare challenging; appointments are postponed, cancelled, changed to virtual or preceded by a virtual call.
pandemic has made healthcare challenging; appointments are postponed, cancelled, changed to virtual or preceded by a virtual call.
Many of us also have lives with limited social connections already so an additional barrier makes life that more challenging. The fear of being affected by COVID-19 when already affected by Pompe Disease, due to risk for respiratory illness, is real.”
- Brad Crittenden
Congenital Myopathies | Alison Engel-Yan and Gavi Engel-Yan
“I have three amazing daughters, 13 year old twins and a 10 year old, Gavi, who has a rare  neuromuscular condition called Nemaline Myopathy and is at risk for severe COVID-19 complications due to weak respiratory muscles.
neuromuscular condition called Nemaline Myopathy and is at risk for severe COVID-19 complications due to weak respiratory muscles.
We are worried about risk for our senior parents, but we also worry about Gavi. Vulnerable kids have been entirely forgotten in pandemic responses - not yet eligible for vaccines and reliant on many different caregivers who get no priority for vaccines. We have night nurses and school nurses coming in and out of the house. Gavi also loves and needs to be in school when school is in - schools must be safe for everyone but classes are too big, testing and tracing are inadequate. We know there is risk but she has the same rights to be in school as all kids so we take as many precautions as are within our control. And we worry.”
- Alison Engel-Yan
“COVID-19 affects me more because I have Nemaline Myopathy. Life during the pandemic is boring. I can do less activities than my sisters - like skating and hanging out with my friends outside. When I am outside, it is colder for me because I can’t move around and keep myself warm in my wheelchair.”
- Gavi Engel-Yan
Congenital Myopathies | Sarah Szmidt and Rebekkah Baldwin-Sheldon
“Rebekkah has Distal Arthrogryposis Multiplex Congenita. She has been affected directly by  the COVID-19 pandemic by the lack of access and the ability to attend primary and specialist appointments. She was scheduled to have surgery in Spring 2020 and is still waiting. The presence of COVID-19 has shifted our awareness to those with disabilities and health issues but it still hasn’t allowed families the ability to get proper resources to live normal lives.
the COVID-19 pandemic by the lack of access and the ability to attend primary and specialist appointments. She was scheduled to have surgery in Spring 2020 and is still waiting. The presence of COVID-19 has shifted our awareness to those with disabilities and health issues but it still hasn’t allowed families the ability to get proper resources to live normal lives.
As a front-line worker, Rebekkah’s access has further declined due to my need to stay working. Taking time off work is doubly difficult: I either take time away from work and face decreased income or go to work and not be able to schedule appointments and care. The result has me being her direct and only caregiver while working full time.
While this young woman regularly experiences pain and is affected with mobility, it doesn’t deter her happy-go-lucky personality that beams with smiles and cheer for everyone she meets. Five years ago, I was told that she would not walk. Five years ago, I was determined to help her be the best version of her that she could. This past year she continues to meet milestones: hopping, jumping, riding a bike, swimming, learning to skate, Taekwon Do, and even participating in a 1 KM walk locally. It hasn’t been easy and there will always be hurdles, but this little girl is my hero, my reason, and my example of true strength and perseverance.”
- Sarah Szmidt
Muscular Dystrophies | Nadia Corapi
“Living in the midst of a pandemic with a physical debilitating disorder like Limb Girdle  Muscular Dystrophy definitely presents new challenges. Winters have always been challenging with mobility issues and accessing things safely, but a pandemic definitely it ups the ante.
Muscular Dystrophy definitely presents new challenges. Winters have always been challenging with mobility issues and accessing things safely, but a pandemic definitely it ups the ante.
I have found the inability to access my Pilates class as a huge set back. Pilates has helped keep me strong, more flexible and was an important part of my mental health. Removing this necessary aspect of my life has left me feeling frustrated, limited to physical purposeful movement and a little fearful of what new physical limitations this may cause.
Social interaction is definitely a necessary human need. Missing family, not being able to support one another in difficult times such as grieving has left me feeling removed and disconnected. x`x Although I am blessed to have an amazing husband and great kids - this pandemic has taken its toll on me in many ways: physically, emotionally, and mentally.”
- Nadia Corapi
Congenital Muscular Dystrophies | Johanne Boudreault
“Since May 2019, I have gone from a known degenerative disease diagnosis, Congenital  Myopathy, to a rare non-degenerative orphan disease: Bethlem Myopathy. In 2020, three issues turned my life upside down in COVID times. First, I turned 65 which meant I was in the high-risk group for COVID on top of my neuromuscular condition. Then, I fell and broke my arm.Third, my caregivers, not knowing anything about this disease and the effects that it could have on my body, refused to treat me. When I was hospitalized, the COVID-19 pandemic complicated things: I spent 14 days in isolation in the hospital without orthopaedic treatment. I witnessed first-hand the many challenges that healthcare staff had to face and deal with in the pandemic. I felt the strain on orthopedic care, as I had to fight for a few essential treatments so that I could use my arm and start walking again. Denying me these treatments was like nailing me to a wheelchair. Today, I continue to fight for my right to autonomy in the pandemic."
Myopathy, to a rare non-degenerative orphan disease: Bethlem Myopathy. In 2020, three issues turned my life upside down in COVID times. First, I turned 65 which meant I was in the high-risk group for COVID on top of my neuromuscular condition. Then, I fell and broke my arm.Third, my caregivers, not knowing anything about this disease and the effects that it could have on my body, refused to treat me. When I was hospitalized, the COVID-19 pandemic complicated things: I spent 14 days in isolation in the hospital without orthopaedic treatment. I witnessed first-hand the many challenges that healthcare staff had to face and deal with in the pandemic. I felt the strain on orthopedic care, as I had to fight for a few essential treatments so that I could use my arm and start walking again. Denying me these treatments was like nailing me to a wheelchair. Today, I continue to fight for my right to autonomy in the pandemic."
- Johanne Boudreault
Hereditary Peripheral Nerve Disorders | Wes Damon
“Living with Charcot-Marie-Tooth Disease or as some call it, Hereditary Motor Sensory  Neuropathy, during the COVID-19 pandemic has been difficult to say the least. I have been shut off from a lot – a lot more than usual life with a rare condition. The simple things like going to a coffee shop or going for a walk through the mall are now gone. For me this was my only way to get out as it was easier on my legs to walk. It has been hard not only on my body but also on my mind – on my emotional and mental health. I always knew living with a rare condition like CMT made me appreciate the little things, but now more than ever I appreciate all the little things in life.”
Neuropathy, during the COVID-19 pandemic has been difficult to say the least. I have been shut off from a lot – a lot more than usual life with a rare condition. The simple things like going to a coffee shop or going for a walk through the mall are now gone. For me this was my only way to get out as it was easier on my legs to walk. It has been hard not only on my body but also on my mind – on my emotional and mental health. I always knew living with a rare condition like CMT made me appreciate the little things, but now more than ever I appreciate all the little things in life.”
- Wes Damon
Hereditary Peripheral Nerve Disorders | Traci Williams
“When the pandemic started, it was the middle of winter… as someone with CMT, I tend to  “hibernate” in the winters, so I had a well-stocked freezer and much that I needed. I already worked from home and in a way was prepared. In a sense, I felt I was made for this, having had to adapt all my life to various situations. I make sure I am okay on all levels, get the rest I need, connect with people regularly, attend church online, let my doctor know if anything is happening, have what I need delivered, recognize that this is a difficult time, take in the facts, and then strive to find bits of joy (for example, I play music).
“hibernate” in the winters, so I had a well-stocked freezer and much that I needed. I already worked from home and in a way was prepared. In a sense, I felt I was made for this, having had to adapt all my life to various situations. I make sure I am okay on all levels, get the rest I need, connect with people regularly, attend church online, let my doctor know if anything is happening, have what I need delivered, recognize that this is a difficult time, take in the facts, and then strive to find bits of joy (for example, I play music).
I am an introvert, so in some ways, I do not have as much stress… it was always an ordeal to get outside with my disease, so not having to or finding other ways to do what I need to do has taken some pressure off of me.”
- Traci Williams
Autoimmune Mediated Myopathies | Elaine Dicken
“Living with a rare condition, Inclusion Body Myositis, during a pandemic has meant that I  could not physically see some of my family and friends for almost a year now. I’m tired of eating my own cooking. I don’t get enough exercise or fresh air because I’m not even grocery shopping as often, particularly in the winter. There are some benefits: telephone doctor appointments to renew prescriptions and learning new technology skills. I was skeptical about virtual physiotherapy appointments, but it has worked quite well. In spite of the challenging 11 months, I’m thankful I have hobbies that keep me connected like volunteering with Myositis Canada and participating in the Muscular Dystrophy Canada virtual network meetings.
could not physically see some of my family and friends for almost a year now. I’m tired of eating my own cooking. I don’t get enough exercise or fresh air because I’m not even grocery shopping as often, particularly in the winter. There are some benefits: telephone doctor appointments to renew prescriptions and learning new technology skills. I was skeptical about virtual physiotherapy appointments, but it has worked quite well. In spite of the challenging 11 months, I’m thankful I have hobbies that keep me connected like volunteering with Myositis Canada and participating in the Muscular Dystrophy Canada virtual network meetings.
- Elaine Dicken
Muscular Dystrophies | Brad Miller
“Living with a rare condition, Becker Muscular Dystrophy, during the COVID-19 pandemic has  been tough. I have to take careful precautions when venturing out in the community.
been tough. I have to take careful precautions when venturing out in the community.
A truly helpful thing I've taken advantage of is the option of scheduling grocery pick-ups, which does help limit my exposure to larger crowds.
Like most, the inability to spend time with friends and family has been the most difficult. I hope one day things will get back to normal.”
- Brad Miller
Muscular Dystrophies | Alfred Breton-Pare and Eloi
“For a family impacted by a neuromuscular disorder, in our case Duchenne Muscular Dystrophy,  the pandemic exacerbated the challenges we are already facing. For our son Éloi, it meant delays in medical assessments and for some treatments. With the confinement, we have also witnessed a pause in clinical research activities and restrictions to travel to hospitals located in other provinces. With precious time passing by, it could mean losing eligibility to an investigational treatment (either by aging, loss of physical capabilities or end of recruitment). With the vaccination currently ongoing, let’s hope all activities will return soon to a normal setting for every families within our community.”
the pandemic exacerbated the challenges we are already facing. For our son Éloi, it meant delays in medical assessments and for some treatments. With the confinement, we have also witnessed a pause in clinical research activities and restrictions to travel to hospitals located in other provinces. With precious time passing by, it could mean losing eligibility to an investigational treatment (either by aging, loss of physical capabilities or end of recruitment). With the vaccination currently ongoing, let’s hope all activities will return soon to a normal setting for every families within our community.”
- Alfred Breton-Pare
“COVID-19 has certainly limited my recreational and social activities. We could not meet with family and friends like we used too. I also felt isolated from my teachers and classmates during the confinement. It was a great news when schools re-opened. Despite being restricted to only one “bubble”, I realized however that it facilitated creating new friendships.”
- Eloi




 I also have Becker’s Muscular Dystrophy. I wanted to share my story with dedicated donors, like you, because you’re having a profound impact on the lives of so many people affected by neuromuscular disorders – in ways that you may not even realise. And for that you deserve much gratitude and thanks.
I also have Becker’s Muscular Dystrophy. I wanted to share my story with dedicated donors, like you, because you’re having a profound impact on the lives of so many people affected by neuromuscular disorders – in ways that you may not even realise. And for that you deserve much gratitude and thanks.

 meant. But the doctor told us that my nervous system is slower and doesn’t work as well as other kids’.
meant. But the doctor told us that my nervous system is slower and doesn’t work as well as other kids’. learn how to dance so my mom signed me up for dance classes. She didn’t want to wait because one day I might be in my wheelchair full-time and dance classes would be very different.
learn how to dance so my mom signed me up for dance classes. She didn’t want to wait because one day I might be in my wheelchair full-time and dance classes would be very different.