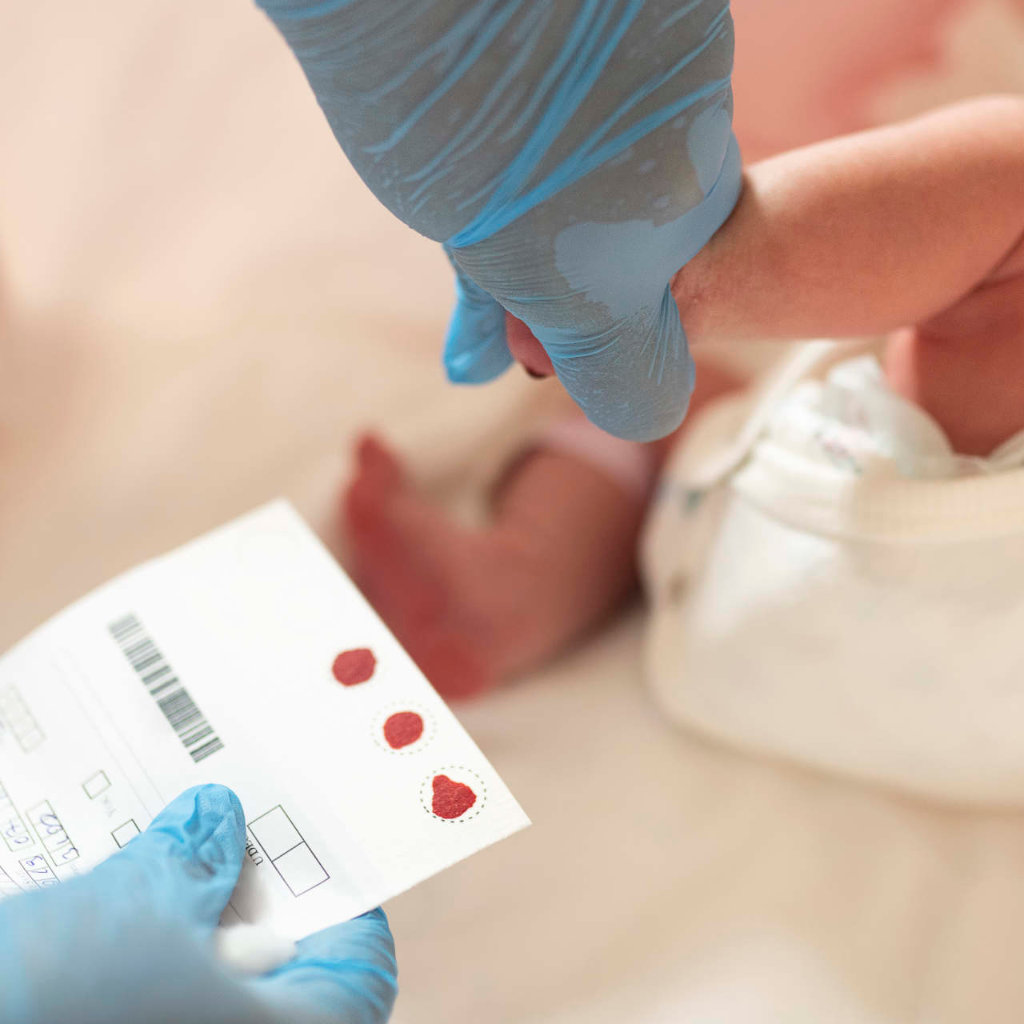
Toronto, Ontario – Muscular Dystrophy Canada (MDC) is proud to announce an investment of $832,766 to fund nine new clinical and translational science research projects in 2023 through the MDC neuromuscular disorder (NMD) research grant competition, a dedicated Canadian source of funding for neuromuscular research.
“Muscular Dystrophy Canada is a leader in funding ground-breaking research for all neuromuscular disorders. This does two things: first, it lets us advance the global understanding of NMDs and the advancement of more treatments, therapies, and potential cures. And second, our involvement with these research projects means we can get people the most up-to-date information they need,” stated Stacey Lintern, CEO, Muscular Dystrophy Canada.
Drawing on the expertise of Canadian and international reviewers, including neurologists, researchers, allied health care professionals, and people with lived experience, the top projects were identified for funding.
“We held a rigorous peer-review process, and as a result, the projects selected for funding are diverse and potentially high-impact research. The projects cover different neuromuscular disorders and aspects of diagnosis, clinical care, and management and evaluate the impact on muscle function and systems outside of muscles, as well as quality of life,” said Dr. Homira Osman, Vice President, Research and Public Policy, Muscular Dystrophy Canada. She added, “supporting such projects not only contributes to the knowledge base, but it strengthens the Canadian neuromuscular research infrastructure and brings us one step closer to cures.”
The projects submitted this year for consideration were of remarkable quality, and we thank each and every team for their interest in doing this important work. MDC relies on the generosity of Fire Fighters, donors and volunteers to invest in life-changing research, and is honoured to fund the exceptional and bright researchers, clinicians and academics who will lead these nine new projects taking place in hospitals and universities across Canada.
Learn more “MDC funds nine new projects to propel research forward and break down barriers”




 Stacey Lintern
Stacey Lintern Perry Esler
Perry Esler