Neuromuscular Disease Network for Canada Awarded 5-Year Grant from CIHR-IMHA and Funding from MDC to Strengthen Canadian Neuromuscular Research and Care
For Immediate Release: June 21, 2023
Toronto, ON – The Neuromuscular Disease Network for Canada (NMD4C) has received a network grant from the Canadian Institutes of Health Research – Institute of Musculoskeletal Health and Arthritis (CIHR-IMHA), providing funding of $200 000 per year for five years – with matched funding from Muscular Dystrophy Canada (MDC) – to strengthen the care, research and treatment of neuromuscular diseases (NMDs) for all Canadians. The new grant brings together an expanded group of clinicians, scientists and patient representatives under the leadership of Dr Hanns Lochmüller (Children’s Hospital of Eastern Ontario) and Dr Homira Osman (MDC).
“Since 2020, NMD4C has made remarkable progress in uniting Canada’s neuromuscular community. In these rare diseases, it’s crucial that we work together. We’re delighted that we have grown to more than 500 members spanning multiple disciplines, sectors, and areas of expertise. The network has made tremendous strides in building capacity through training and education, providing leadership and advocacy to improve access to approved novel treatments, and strengthening research resources and infrastructure,” explains Dr. Hanns Lochmüller, Senior Scientist, CHEO Research Institute and Professor of Neurology, University of Ottawa. “But there’s still so much more to be done. New scientific challenges and opportunities mean that networking across Canada and the globe is even more important. This new funding will allow us to expand our supportive, collaborative, networked community of neuromuscular stakeholders, bringing together an unparalleled concentration of NMD expertise to provide a Canada-wide platform for communication, collaboration, and best-practice sharing. We’re very grateful to CIHR-IMHA and MDC for the funding that allows us to continue our work. We have some really exciting plans for this next phase of the network, particularly for our young doctors and researchers, and we can’t wait to get started.”
Building on the NMD4C’s successful work over the past three years, this new grant will enable an ambitious new program of research, networking, and clinical transformation to address emerging challenges in the field of NMDs. With a total of 67 named co-investigators from across the country bringing in their wider teams of researchers, this is one of the largest networking projects in the rare disease field in Canada. Matching funds are being provided by Canada’s leading NMD advocacy organization and partner on the grant MDC, for a combined total amount of $400 000 per year of network funding.
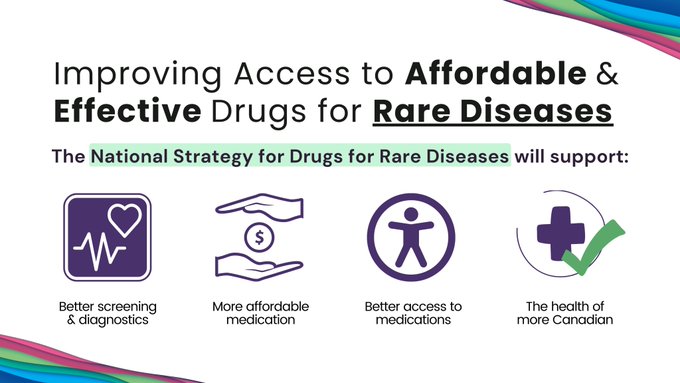
“We greatly value our longstanding partnership with the CIHR, and are deeply thankful to our many partners, Fire Fighters, donors, volunteers and supporters who generously give so that we can fund the vital work of NMD4C,” said Dr Homira, Osman, Vice-President, Research and Public Policy, Muscular Dystrophy Canada. “In addition to offering matched funding, our role in the new grant will be to ensure evidence is translated into practices and policies that will make a tangible difference in the lives of Canadians affected by neuromuscular disorders. Aligned with our recently unveiled Breaking Down Barriers five-year strategic direction, we will partner closely with researchers, clinicians, and the neuromuscular community to enhance proactive and collaborative approaches focused on strengthening infrastructure, enhancing capacity, establishing centers of excellence, and facilitating the dissemination of research outcomes.”
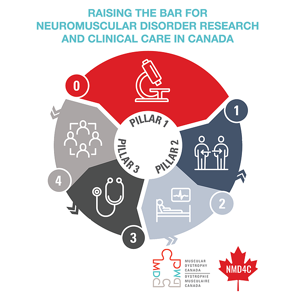
This new grant unites people with lived experience, knowledge users, clinicians, and researchers to execute a new research plan with the following objectives:








 Stacey Lintern
Stacey Lintern Perry Esler
Perry Esler


