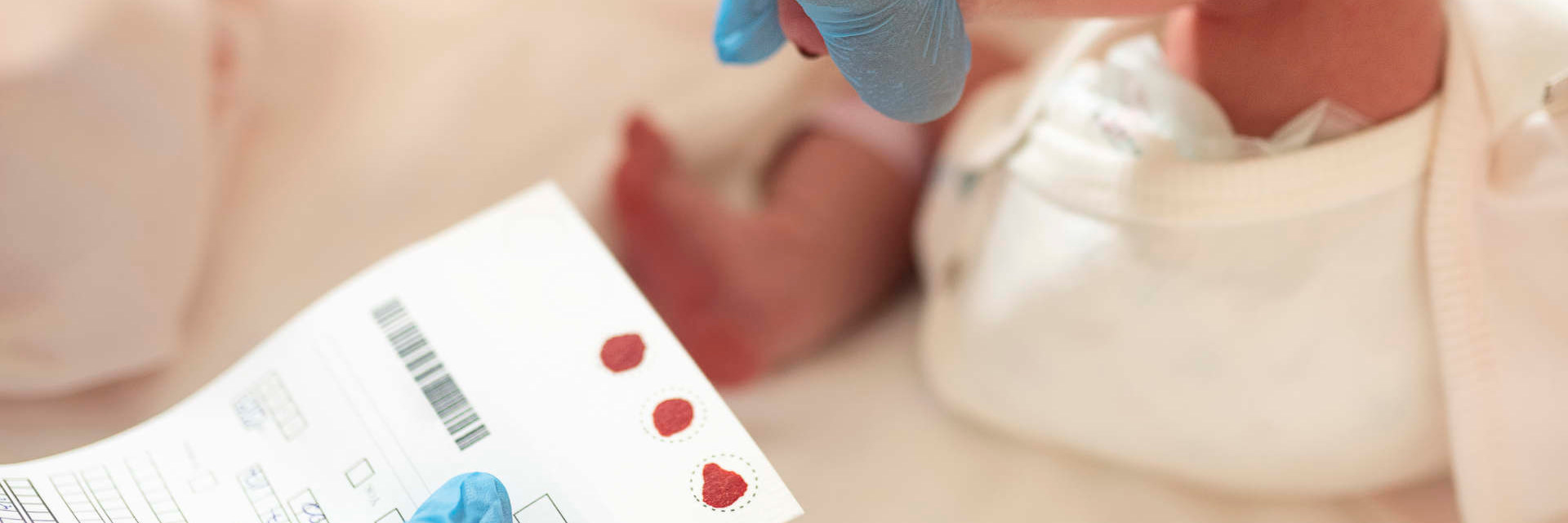
- Spinal Muscular Atrophy (SMA) is the leading cause of genetic infant death.
- Early diagnosis is imperative to halt irreversible motor neuron loss and disease progression.
Toronto, ON, November 18, 2021 – Muscular Dystrophy Canada (MDC) today provided $414,000 to assist the Maritimes in evaluating methodology that would support readiness to add spinal muscular atrophy to their newborn screening panel. The Maritime Newborn Screening Program (MNSP), based at the IWK Health Centre but servicing Nova Scotia, New Brunswick and Prince Edward Island, was awarded the funds through a collaborative initiative between MDC and Novartis Pharmaceuticals Canada Inc. (Novartis).
“In a neuromuscular disorder like SMA, where time is of the essence, early diagnosis and prompt access to treatments are critical to achieving the best possible outcomes. Sadly, most provinces are not yet screening for this treatable disorder,” said Stacey Lintern, CEO, Muscular Dystrophy Canada. “I thank the healthcare leaders at MNSP, Novartis, the SMA community and MDC’s dedicated Board of Directors, Fire Fighters, clients, donors and supporters, for getting us another step closer to ensuring all Canadian newborns are screened for SMA.”
Newborn screening is a test done for babies shortly after birth to look for treatable diseases that usually show no symptoms in the newborn period.
In order to ensure an evidence-based approach to funding, MDC and Novartis commissioned a readiness assessment to evaluate the feasibility of adding SMA to all provincial and territorial screening panels. Proposals were then evaluated by an independent international peer review committee under MDC’s guidance. Further details on the needs and readiness assessment, project selection, peer review, community advisory committees and video overview can be found here: muscle.ca/newborn-screening/.
“We’re honoured to partner on work that will bring lasting impact to the SMA community and healthcare systems across Canada,” said Andrea Marazzi, Country Pharma Organization Head, Novartis Pharmaceuticals Canada. “Time to diagnosis is crucial in SMA and we applaud the Maritimes for their commitment to prioritize screening at birth.”
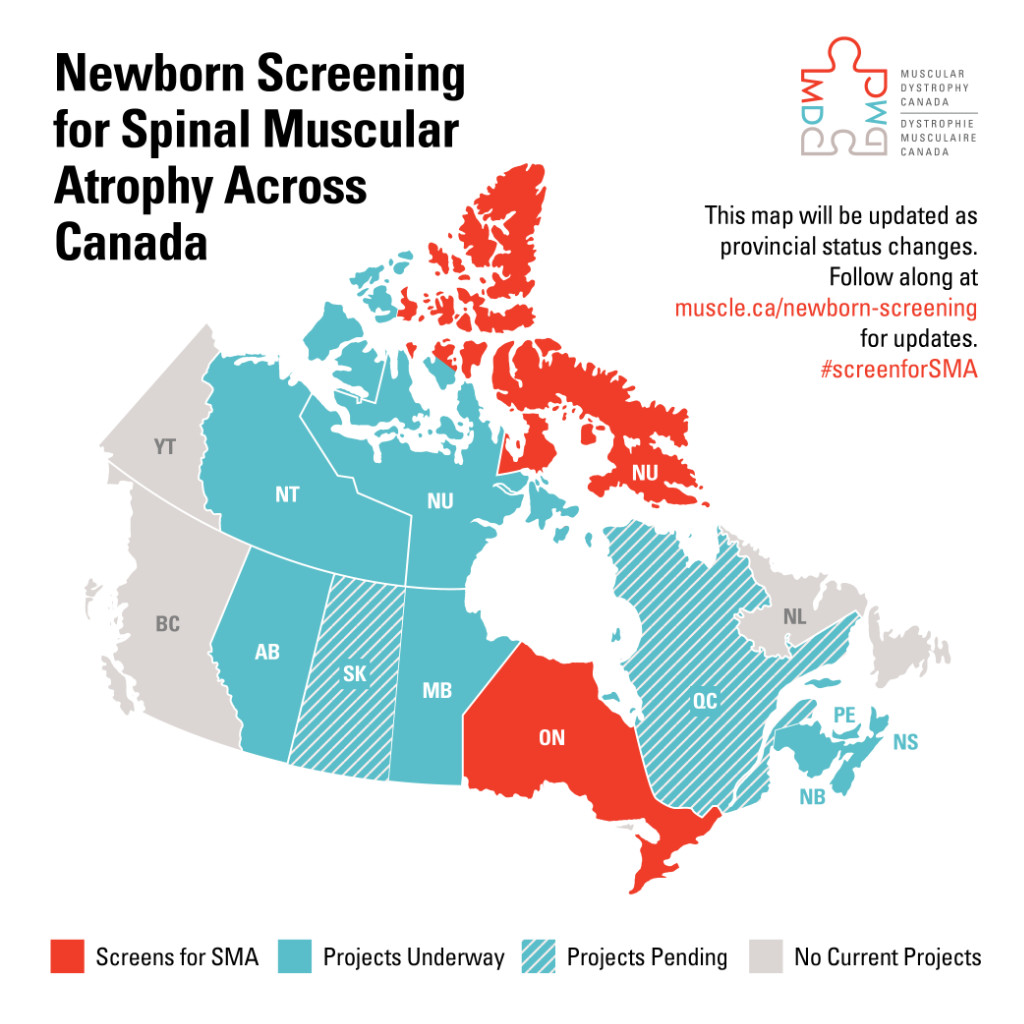
Newborn screening for SMA currently only takes place in Ontario, but projects are now underway in Alberta and Manitoba as well. Future phases of the national collaboration will include additional project funding, evaluation of projects and knowledge transfer, and exchange with stakeholders to ensure policy adoption across all provinces and territories.
About Muscular Dystrophy Canada
Muscular Dystrophy Canada’s mission is to enhance the lives of those affected with neuromuscular disorders by continually working to provide ongoing support and resources while relentlessly searching for a cure through well-funded research. To learn more about Muscular Dystrophy Canada, please explore our website or call our toll-free number at 1-800-567-2873.
– 30 –
For more information:
Heather RiceDirector, Marketing and Communications
heather.rice@muscle.ca
902-440-3714