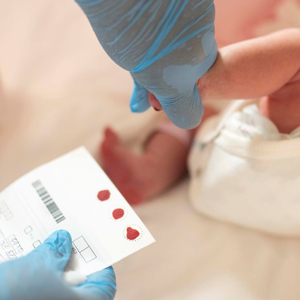
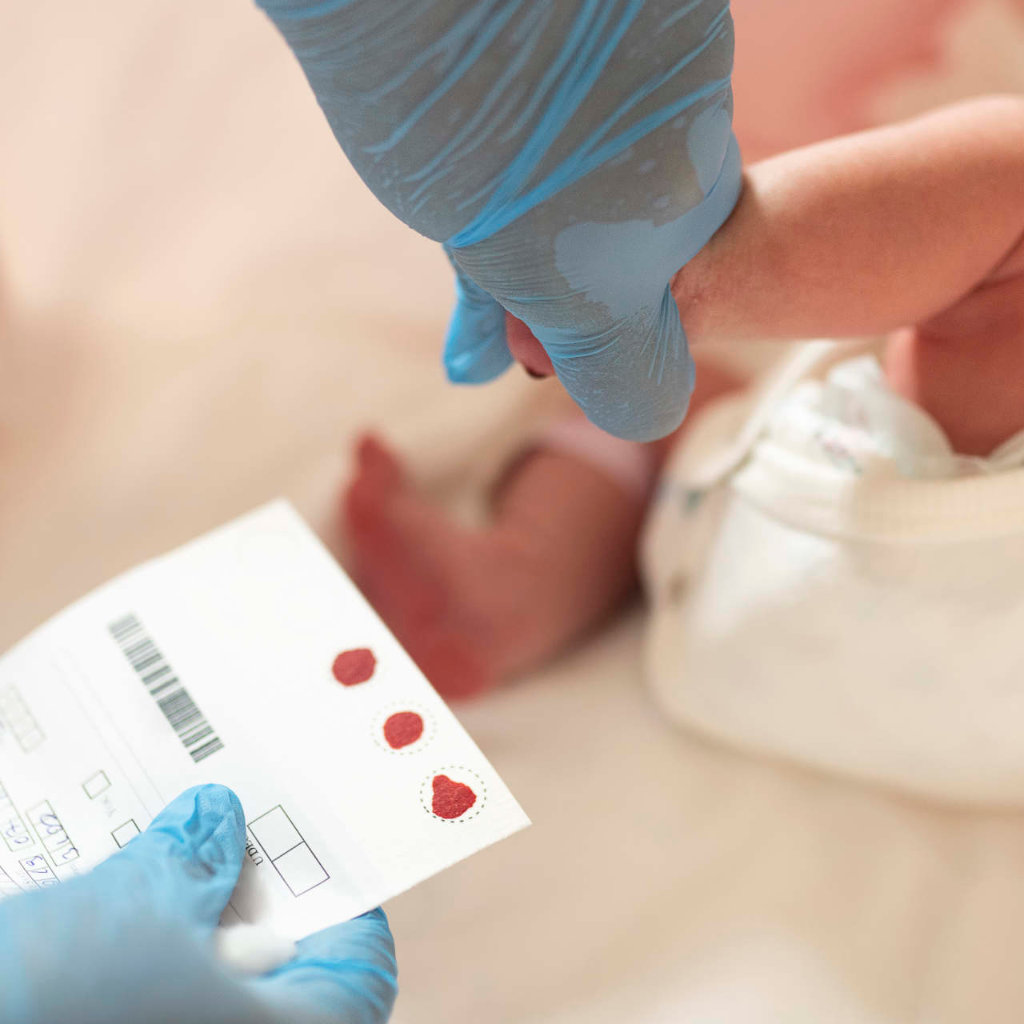
Danielle has defied all odds throughout her life. As a world-record holding Paralympian, Danielle challenged herself to win a medal, and not only did she win one, but over the course of two Olympic games, she won seven. Danielle is a published book author and engages many as a motivational speaker at events nationwide. She’s a proud mom of five and has earned an honourary doctorate. Danielle also has spinal muscular atrophy (SMA).
When she was two years old, Danielle was diagnosed and her lifelong connection to Muscular Dystrophy Canada began.
Because of our donors, Muscular Dystrophy Canada has been able to stand shoulder to shoulder with Danielle and her family throughout every stage of her neuromuscular journey, from supporting them as they navigated through some difficult questions, helping to provide vital equipment and working together to ensure the systems around Danielle were set up to ensure her success.
Please make a gift today and help families, like Danielle’s, get the answers and support they so desperately need.
“Not only did Muscular Dystrophy Canada provide emotional support to my family, but throughout my childhood they also provided support with equipment, advocacy, and navigating life with a neuromuscular disorder,” Danielle says. “Growing up, I had many questions: Would I be able to have kids? Would I pass this disorder on to my children? What would my prognosis look like? No matter what question or fear I had, I was always able to turn to Muscular Dystrophy Canada.”
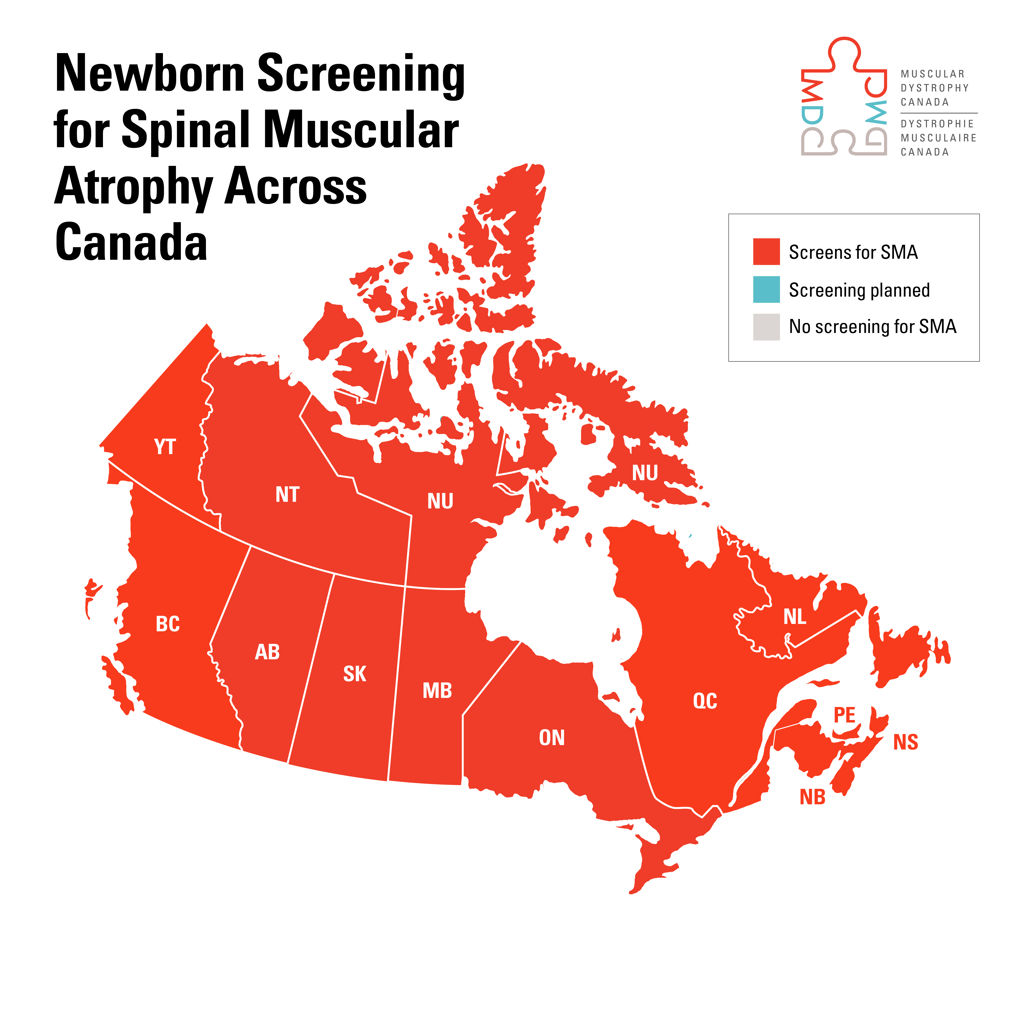
Today, Danielle is thriving. But life isn’t always easy. Because receiving a neuromuscular diagnosis can be complex, Danielle received a new diagnosis recently. The good news is that this was possible because of our donors. Your investments ensured research continued to accelerate and change lives.
“Thanks to Muscular Dystrophy Canada’s donors, I have been able to finally get my official diagnosis of spinal muscular atrophy – lower extremities dominant (SMA-LED), after 10 years of genetic testing” Danielle says. “Thanks to this investment, I was also able to learn that my condition is genetic and there was a 50/50 chance it would be passed onto my children.”
Originally told she wouldn’t pass on her disorder, Danielle’s middle son, Samson was recently diagnosed with spinal muscular atrophy, just like his mom.
The path ahead for Danielle and Samson isn’t a straight line but is made easier because of Muscular Dystrophy Canada’s support, programs and services.
Our team is at the end of each phone call. We will answer every email, and we will help ensure Danielle, her family and everyone living with a neuromuscular disorder has every opportunity to meet their own goals, whatever they may be.