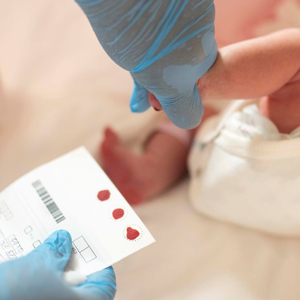
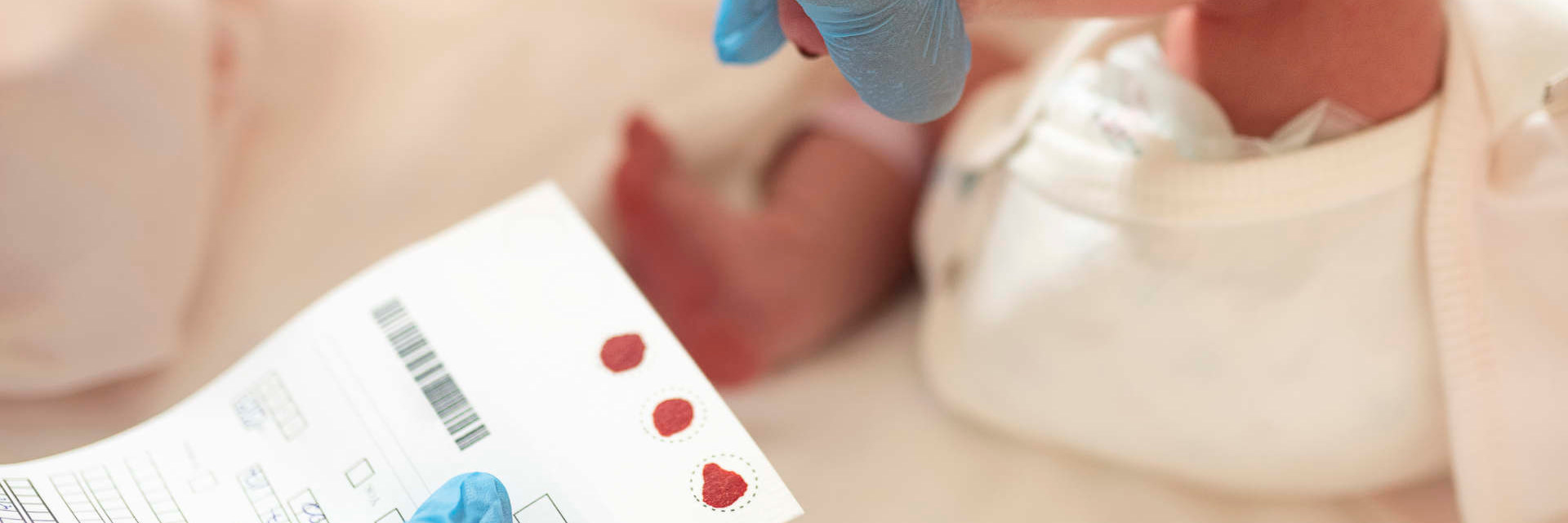
Muscular Dystrophy Canada is thrilled to announce that after three years of working with each Canadian province and territory, all babies born in Canada can now be tested for spinal muscular atrophy (SMA). This milestone means infants diagnosed with SMA, the most fatal genetic disorder in children under two years of age, can receive life-changing treatment before symptoms even develop. It also marks the first neuromuscular condition to be added to screening panels across the country.
“Early diagnosis and effective treatment are critical to achieving the best possible outcomes for babies born with this neuromuscular condition,” said Dr. Pranesh Chakraborty , Chief of the Department of Pediatrics at Children’s Hospital of Eastern Ontario and Chair of the Department of Pediatrics at uOttawa’s Faculty of Medicine. “Historically, most infants and children with SMA would have been diagnosed only after they have developed weakness and respiratory difficulty, at a time when most of their motor neurons have been irretrievably lost. Now, instead of facing life-limiting disability and, in the most severe cases, a life expectancy of less than two years, babies affected by SMA every year in Canada shall be diagnosed within the first weeks of life allowing them to rapidly receive therapy and improved outcomes.”
The addition of SMA to newborn screening panels in all provinces and territories breaks down barriers and inequities families face simply because of where they live. It also acknowledges that screening for rare genetic diseases and access to early care result in positive health outcomes and long-term cost benefits for everyone. This is a tremendous accomplishment to start improving early detection and prevention.
We are so grateful for the partnership with Novartis Pharmaceuticals Canada, who helped us make this a reality across Canada. And a very special thank you to our dedicated community, generous donors and sponsors, Fire Fighter partners, clinicians, clients, and advocates for your unwavering support. We have helped make a life-changing impact on newborns and their families nationwide. We did it together—thank you!
For more information on this and other advocacy initiatives, please reach out through the research Hotline at 1-800-567-2873 ext. 114 or via email at research@muscle.ca.

Was your child diagnosed with SMA through newborn screening?
If so, we offer a specialized program called SMArTrack to help monitor, assess, and provide answers during the first two years of their life. For more information, or to get involved, please reach out to research@muscle.ca.
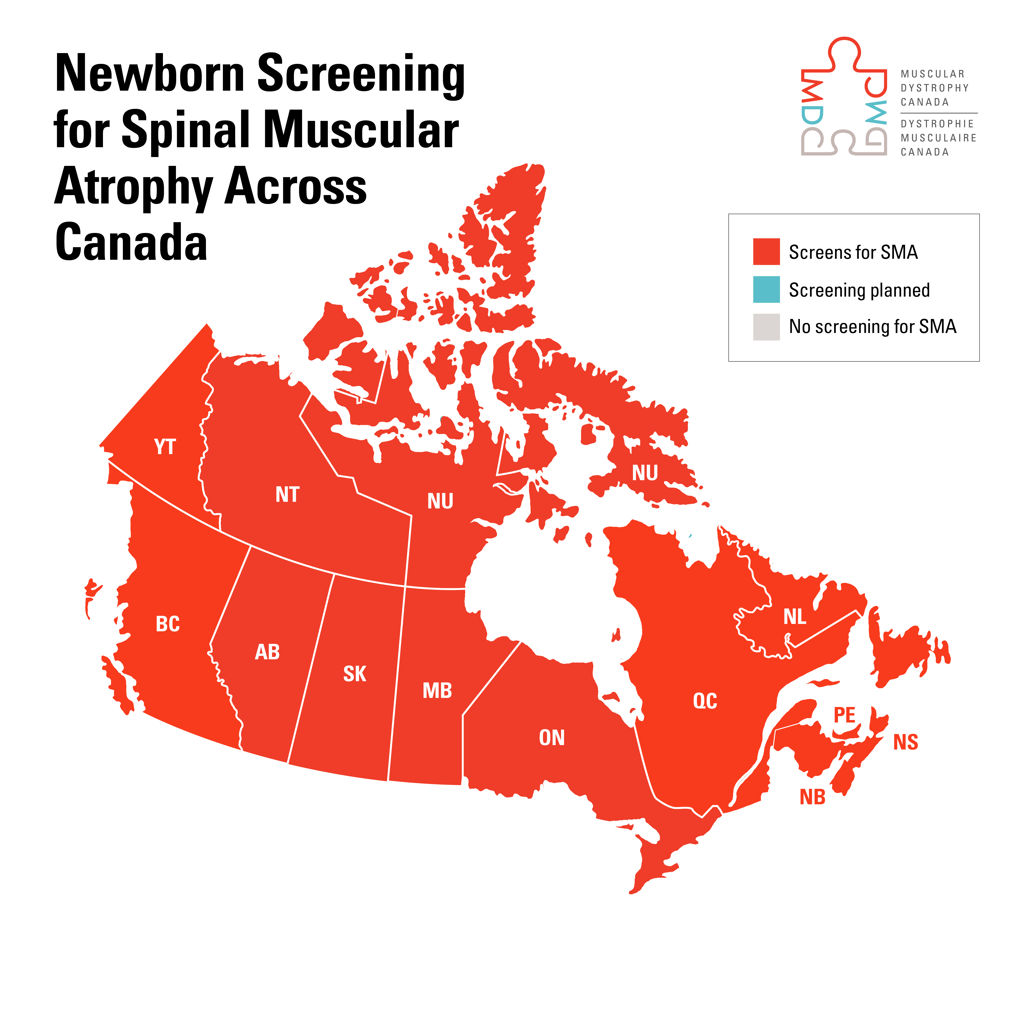
100 percent of Canada is now screening infants for spinal muscular atrophy, a potentially fatal disorder
Muscular Dystrophy Canada and partners deliver on goal outlined in Canada’s Rare Disease Strategy
Toronto, Ontario, Canada, August 20, 2024 – Muscular Dystrophy Canada is thrilled to announce that after three years of working with each Canadian province and territory, all babies born in Canada can now be tested for spinal muscular atrophy (SMA). This milestone means infants diagnosed with SMA, the most fatal genetic disorder in children under two years of age, can receive life-changing treatment before symptoms even develop. It also marks the first neuromuscular condition to be added to screening panels across the country.
“Early diagnosis and effective treatment are critical to achieving the best possible outcomes for babies born with this neuromuscular condition,” said Dr. Pranesh Chakraborty Chief of the Department of Pediatrics at Children’s Hospital of Eastern Ontario and Chair of the Department of Pediatrics at uOttawa’s Faculty of Medicine. “Historically, most infants and children with SMA would have been diagnosed only after they have developed weakness and respiratory difficulty, at a time when most of their motor neurons have been irretrievably lost. Now, instead of facing life-limiting disability and, in the most severe cases, a life expectancy of less than two years, babies affected by SMA every year in Canada shall be diagnosed within the first weeks of life allowing them to rapidly receive therapy and improved outcomes.”
In 2020, only Ontario and the Baffin region in Nunavut screened for SMA at birth. Today, regardless of where in Canada a child is born, they will receive the same screening; and, if SMA is diagnosed, the same healthcare, treatment and opportunity to thrive.
“I could not imagine what our life would look like if my daughter were not given genetic, SMA-testing at birth,” said Taylor Diakew, mother of a 2-year-old with SMA. “Today, thanks to her early diagnosis, and quick access to treatment, she is a happy, healthy little girl who does not exhibit any signs of SMA – she is walking, running, climbing, and talking like any child her age. Thanks to SMA newborn screening, she can live the best life possible.”
The addition of SMA to newborn screening panels in all provinces and territories breaks down barriers and inequities families face simply because of where they live. It also acknowledges that screening for rare genetic diseases and access to early care results in positive health outcomes and long-term cost benefits for everyone.
“It is a tremendous accomplishment to start improving early detection and prevention, one of the goals outlined in Canada’s Rare Disease Strategy. However, this success was only possible because of the willingness of provincial and territorial governments to work alongside Muscular Dystrophy Canada to add SMA to newborn screening. This is a significant step forward, and we hope it leads to the inclusion of other neuromuscular conditions on screening panels,” said Stacey Lintern, CEO, Muscular Dystrophy Canada. “We are grateful for the partnership with Novartis Pharmaceuticals Canada who helped us make this a reality across Canada and for every clinician, researcher, provincial laboratory lead, volunteer, donor, Canadian Fire Fighter, like minded organization, and government member who supported this project.”
“This initiative holds immense value for the entire Canadian neuromuscular and rare disease community, laying the groundwork for future transformative and life-changing initiatives,” said Dr. Homira Osman, VP of Research and Public Policy, Muscular Dystrophy Canada. “There are many progressive neuromuscular disorders where time is of the essence: early diagnosis and prompt access to treatments are critical drivers to achieving the best possible outcomes. Muscular Dystrophy Canada will now leverage the knowledge and findings gained from this initiative to ensure other neuromuscular disorders are included in newborn screening programs nationwide.”
– 30 –
ABOUT MUSCULAR DYSTROPHY CANADA
Muscular Dystrophy Canada’s mission is to enhance the lives of those affected with neuromuscular disorders by continually working to provide ongoing support and resources while relentlessly searching for a cure through well-funded research. To learn more about Muscular Dystrophy Canada, please explore our website or call our toll-free number at 1-800-567-2873.
FOR MORE INFORMATION CONTACT:
Homira OsmanVice-President Research & Public Policy
Muscular Dystrophy Canada
Homira.Osman@muscle.ca
437-912-9037
New Ontario-led project to have national impact on newborn screening
Toronto, ON, March 9, 2022 – Muscular Dystrophy Canada (MDC) is proud to announce funding for a first-of-its-kind project to evaluate the cost-effectiveness of newborn screening (NBS) for spinal muscular atrophy (SMA) in Canada, including early treatment. The innovative national project led by a team at the Children’s Hospital of Eastern Ontario (CHEO) will provide vital evidence for policy and decision makers and is expected to help expedite implementation of screening for SMA across the country.
“Discussions on adding new disorders to NBS panels often come down to cost and cost effectiveness: how much will screening for ‘X’ benefit patients and how does that compare to the health system costs? Unfortunately, Canadian health economic analyses are often not available to help answer this question. Our work will generate important information to support newborn screening policy decision making in Canada,” said Dr. Chakraborty, Medical Director of Newborn Screening Ontario (NSO) and project lead.
Today’s announcement is part of MDC’s collaboration with Novartis Pharmaceuticals Canada Inc. (Novartis) to make newborn screening for SMA a national reality. With Alberta and Saskatchewan recently initiating screening for SMA, the focus is now on full-implementation and providing information to support long-term sustainability of all projects.
“Generating this data will not only provide policy and decision makers the evidence they need to make NBS for SMA a national standard, but will also help foster collaboration and exchange of information across the country and can ultimately inform Canada’s Rare Disease Strategy” said Stacy Lintern, CEO, Muscular Dystrophy Canada. “We’re thrilled to be working with Novartis, committed government leaders, the SMA community and MDC’s dedicated Board of Directors, Fire Fighters, clients, donors and supporters to ensure all Canadians have access to the same healthcare, regardless of where they live.”
Access to newborn screening varies widely from province to province. In a progressive neuromuscular disorder like SMA, early diagnosis and prompt access to treatments lead to the best possible outcomes.
“Relocating during pregnancy opened my eyes to the existence of postal code healthcare in Canada,” said Lindsay Williamson, whose son Mason was diagnosed in 2021 with SMA at one month old. “Had we not moved from Ontario for work, our son’s condition would have been promptly picked up during newborn screening. This blood spot would have eliminated the need for a battery of costly medical tests and mitigated the stress of the diagnostic experience. I have no doubt this project will help guarantee families like ours get the information they need from day one.”
Further information on the impact of newborn screening for SMA and the projects funded to date is available at muscle.ca/services-support/newborn-screening/.
About Muscular Dystrophy Canada
Muscular Dystrophy Canada’s mission is to enhance the lives of those affected with neuromuscular disorders by continually working to provide ongoing support and resources while relentlessly searching for a cure through well-funded research. To learn more about Muscular Dystrophy Canada, please explore our website or call our toll-free number at 1-800-567-2873.
-30-
For more information:
Heather Riceheather.rice@muscle.ca
902-440-3714